|
|
China set to provide first swine flu vaccines
http://edition.cnn.com/2009/HEALTH/09/09/china.swine.flu.vaccine/index.html
(CNN) -- China has developed a vaccine for swine flu and is set to become the first country in the world to begin mass inoculations, but there are concerns over possible side effects, the World Health Organization (WHO) has said.
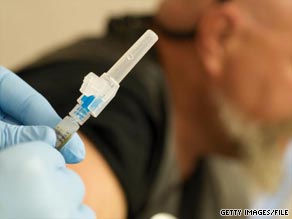
A swine flu vaccine has been approved in China and inoculations could begin in the next few weeks.
WHO spokesman Gregory Hartl told CNN, "We have to be ready for the fact that there might be adverse effects."
"No matter what vaccine you're looking at, sometimes there are extremely rare side effects. We don't even know what those are yet, but they will show up in one in every two or 10 million vaccinated."
Inoculations could begin in the next few weeks, according to the South China Morning Post, before celebrations begin on October 1 to mark the 60th anniversary of the founding of the People's Republic.
The vaccine, PANFLU.1, was developed by Sinovac Biotech Ltd and is suitable for people aged three to 60.
Sinovac says the single-shot vaccine has been approved by China's National Institute for the Control of Pharmaceutical and Biological Products and has obtained the Certificate for the Release of Biological Products. It says more than five million doses will be ready by the end of September.
The South China Morning Post reports Health Minister Chen Zhu as saying that some 200,000 people taking part in the anniversary celebrations will be the first to receive the vaccine.
Others considered to be high priority are students aged five to 19, those with medical conditions, especially chronic respiratory and coronary diseases, and pregnant women. The inoculation program will also target medical staff and key workers, including police officers, soldiers and quarantine officials.
Chen said on Tuesday that there have so far been 5592 recorded cases of H1N1 in China's 31 inland provinces, but no one has yet died from the illness.
"Due to the rising number of cases, especially since late August, we have indeed started seeing some serious cases," he said.
The Post reported that there are plans to vaccinate 65 million people before the end of the year and that Chen admitted the amount of available vaccine was not nearly enough to inoculate the country's population of 1.3 billion people.
The Chinese State Food and Drug Administration (SFDA) last week approved two factories to produce the vaccine -- Sinovac, based in Beijing, and Hualan Biological Engineering, based in Henan -- according to the Post. But it reports the SFDA has announced that "more qualified enterprises which could produce swine flu vaccines" would be licensed by the end of September.
Hartl said that in Europe, several drug firms are set to submit clinical trial data in the next few weeks, with GlaxoSmithKline likely to be among the first.
Weidong Yin, CEO of Sinovac, said last week, "With the support of the Ministry of Health, State SFDA, and Chinese Center for Disease Control and Prevention, Sinovac was able to successfully and rapidly complete the clinical trials and registration process for the H1N1 vaccine."
截图:

|
CNN, first, Flu, set, swine, CNN, first, Flu, set, swine, CNN, first, Flu, set, swine
评分
-
1
查看全部评分
-
|